- Choosing a Research Question
- Formulating Hypotheses
- Identifying/Selecting Endpoints or Outcomes
- Choosing a Study Design
Research Question
A well thought out and specific research question paired with an appropriate study design is crucial to obtaining meaningful results.
Formulating a specific and concise research question can be complicated and will often be a multi-step process. We encourage collaboration with a statistician early and often to ensure the research question is specific and answerable through data collection and statistical analyses.
The below table gives a brief example of this process
| Key Step | Example | |
| Patient Problem | How should I describe a group of people? | Children under the age of 12 have condition X |
| Exposure | What is the main course of treatment I should consider? | If I add drug X into their therapy regimen, what will occur? |
| Comparison | What other treatment options are available? | What occurs if I increase their current dosage of drug X |
| Outcome | What are the patient’s expectations? | Patients want increased symptom control and minimal side effects |
SOURCE
Formulating Hypotheses
Along with a specific and concise research question, hypothesis questions will need to be formed. Research questions in nature are more inquisitive or interrogative. Hypotheses are more predictive and are usually made with preexisting knowledge of the subject. Hypotheses should always be written declaratively instead of interrogatively, like a research question. A hypothesis statement should be developed prior to any data collection.
Do you need help distinguishing between research questions and hypotheses? Visit the following link for in text descriptions of each and a video distinguishing the characteristics between the two provided by Center for Innovation in Research and Testing.
https://cirt.gcu.edu/research/developmentresources/research_ready/quantresearch/question_hypoth
The following link from UCLA provides a nice introduction to hypothesis testing.
http://www.stat.ucla.edu/~rgould/110as02/hypoverview.pdf
Identifying/Selecting Endpoints or Outcomes
Endpoints need to be established immediately after forming a research question as is steers the user in the right direction when establishing whether an outcome will be quantitatively or qualitatively determined.
Study Design
Choosing the Correct Study Design
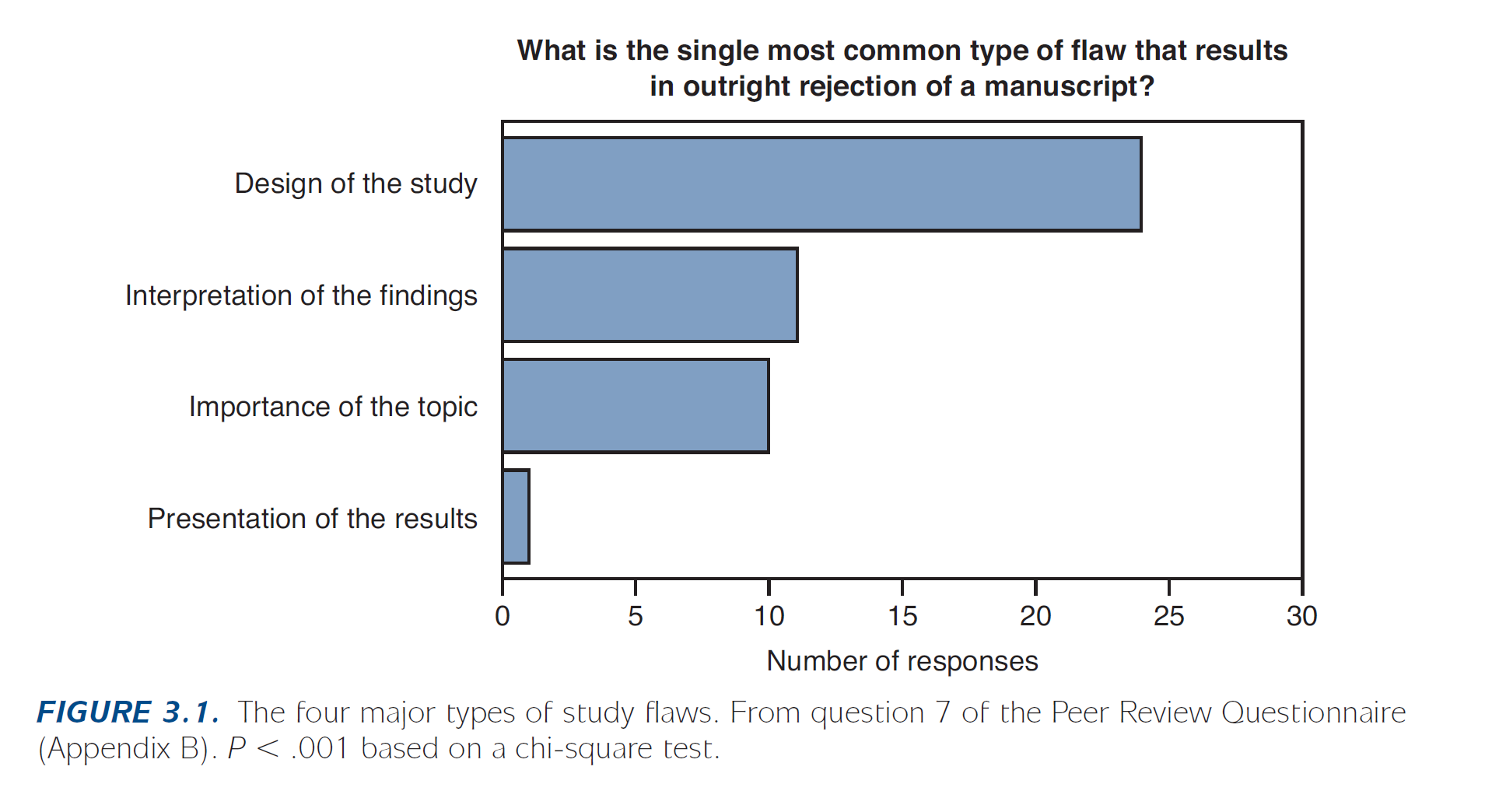
It is critical to choose the correct study design for your question to obtain meaningful results. Because some research questions can often be answered by more than one method/design, the choice of design depends on a variety of considerations, including: availability of time, resources, and data. We encourage a discussion with a statistician early in the research process to ensure a successful study design plan. According to Byrne (Byrne 2017 – Publishing your medical research), study design is the single most common type of flaw that results in outright rejection of a submitted manuscript.
And more importantly, a flawed design may lead to spurious results that cannot answer your research questions.
Basic Study Design Options
The figure below gives basic study design options as well as considerations in selecting each.
Was there an exposure assigned? |
||
|
Yes
|
No
|
|
| 🡻 | 🡻 | |
|
Experimental Study |
Observational Study |
|
|
Comparison group is present
|
No comparison group
|
|
| 🡻 | 🡻 | |
| Analytical study | Descriptive study | |
| 🡻 | ||
|
What is your Direction? |
||
|
Exposure proceeds outcome
|
Outcome proceeds the exposure
|
Exposure and outcome are measured at the same time
|
| 🡻 | 🡻 | 🡻 |
| Cohort Study | Case-Control Study | Cross Sectional Study |
More Information on Outcomes: |
|
Penn State has created an online resource explaining the types of endpoints and how they are used within clinical studies. |
Study Design Reading List |
|
Designing Clinical Research by Hulley et al. (2013) |
|
Fundamentals of Clinical Trials by Friedman et al. (2015) |
|
Evaluating Clinical and Public Health Interventions: A Practical Guide to Study Design and Statistics by Katz (2010) |
|
Basic Statistics for the Health Sciences by Kuzma and Bohnenblust (2004) |
|
How to Write a Lot: A Practical Guide to Productive Academic Writing by Silvia (2007) |
|
Medical Uses of Statistics by Bailar and Hoaglin (2009) |
|
Statistics with Confidence: Confidence Intervals and Statistical Guidelines by Altman et al. (2000) |
|
How to Report Statistics in Medicine: Annotated Guidelines for Authors, Editors, and Reviewers by Lang and Secic (2006) |
|
The Man Who Discovered Quality: How W. Edwards Deming Brought the Quality Revolution to America – The Stories of FORD, XEROX, and GM by Gabor (1992) |
|
Epidemiology by Gordis (2013) |
|
Essentials of Medical Statistics by Kirkwood and Sterne (2003) |
|
Clinical and Translational Science: Principles of Human Research by Robertson and Williams (2016) |
|
Statistical Modeling for Biomedical Researchers: A Simple Introduction to the Analysis of Complex Data by Dupont (2009) |
|
Modern Epidemiology by Rothman et al. (2012) |
|
Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating by Steyerberg (2010) |
|
Encyclopedia of Biostatistics: 8-Volume Set by Armitage and Colton (2005) |
|
Essentials of Writing Biomedical Research Papers by Zeiger (1999) |
|
Statistical Issues in Drug Development by Senn (2008) |
|
Experimental Design for Biologists by Glass (2014) |
|
Experimental Design for the Life Sciences by Ruxton and Colegrave (2010) |
|
Thinking, Fast and Slow by Kahneman (2013) |
Resources: |
|
A brief guide to the different study types and a comparison of advantages and disadvantages: https://www.cebm.net/2014/04/study-designs/
An overview of study design options: https://www.cebm.net/wp-content/uploads/2014/06/CEBM-study-design-april-20131.pdf
Types of clinical study designs: https://research.library.gsu.edu/c.php?g=115595&p=755213
A review of novel methods and technologies for 21st-century clinical trials https://www.ncbi.nlm.nih.gov/pubmed/25730665
Research designs in sports physical therapy https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3474303/
Alternative and Emerging Study Designs |
Contact Us
Williams Building
University of Utah Research Park
Williams Building, 1st floor
295 South Chipeta Way
Salt Lake City, Utah
Map
Parking: During construction, you may park on the bottom floor of the south parking structure.
Contact
Camie Derricott
Phone: 801-587-5212
Fax: 801-581-3623
Acknowledging the SDBC
Please use the following text to acknowledge the CTSI Study Design and Biostatistics Center:
"This investigation was supported by Translational Research: Implementation, Analysis and Design (TRIAD), with funding in part from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UM1TR004409. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health."
"This investigation was supported by the Study Design and Biostatistics Center (SDBC), with funding in part from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UM1TR004409. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health."